Abstract
Background: Sickle cell disease (SCD) is an inherited disorder in which sickle hemoglobin (HbS) polymerization causes red blood cell sickling, resulting in complications such as chronic hemolytic anemia, vaso-occlusion, end-organ damage, and decreased quality of life. Voxelotor is a first-in-class HbS polymerization inhibitor approved in the US for the treatment of SCD in patients aged ≥4 years. The pivotal phase 3 HOPE and phase 2a HOPE-KIDS 1 trials demonstrated the safety and efficacy of voxelotor in increasing hemoglobin (Hb) and decreasing hemolytic markers.
Objective: Here we report Hb response, patient-reported quality-of-life (QOL) outcomes, and safety data from the ongoing ActIVe study (NCT04400487), a phase 4, multicenter, open-label study evaluating the effect of voxelotor on physical activity, sleep quality, and overall well-being in patients with SCD.
Methods: Patients aged 12 to 55 years with SCD received voxelotor 1500 mg/day for up to 24 weeks across 10 US sites. QOL assessments were completed during baseline (BL) run-in before the start of voxelotor. Hb levels were measured via laboratory assessments at BL and at weeks 2, 12, and 24. The proportion of patients with any increase in Hb from BL was noted. Patient-reported outcomes were evaluated using the Patient Global Impression of Change (PGI-C) scale, the National Institutes of Health Patient-Reported Outcome Measurement Information System-43 (PROMIS-43) for adults (>17 years), PROMIS-37 for adolescents (≤17 years), and a 10-point numeric rating scale (NRS) for pain intensity. A minimum response deemed meaningful was interpreted at the group level as a ≥5-point change from BL (CFB) in PROMIS domains and a ≥2-point change on the NRS. Clinicians' perceptions of the impacts of voxelotor on patients' overall health status were captured using the Clinical Global Impression of Change (CGI-C). All treatment-emergent adverse events (TEAEs) were recorded from the time of consent/assent to 28 days after the last dose of voxelotor.
Results: A total of 25 patients were enrolled, with a median (range) age of 20.0 (12-51) years; 64.0% of patients were female and 92.0% were Black or African American. Two (8.0%) patients permanently discontinued voxelotor due to non-compliance and loss to follow-up. A total of 14 of 18 (78%) patients had an increase in Hb at week 24; the mean (SD) increase was 0.53 (0.839) g/dL.
Clinicians reported an improvement in health status among 84.6% (11/13) of patients at week 24: 7.7% (1/13) were "very much improved," 46.2% (6/13) were "much improved," and 30.8% (4/13) were minimally improved; 2 patients had no change. At week 24, 92.9% (13/14) of patients self-reported improved health status: 21.4% (3/14) were "very much improved," 42.9% (6/14) were "much improved," and 28.6% (4/14) were minimally improved; 1 patient reported minimally worse health status.
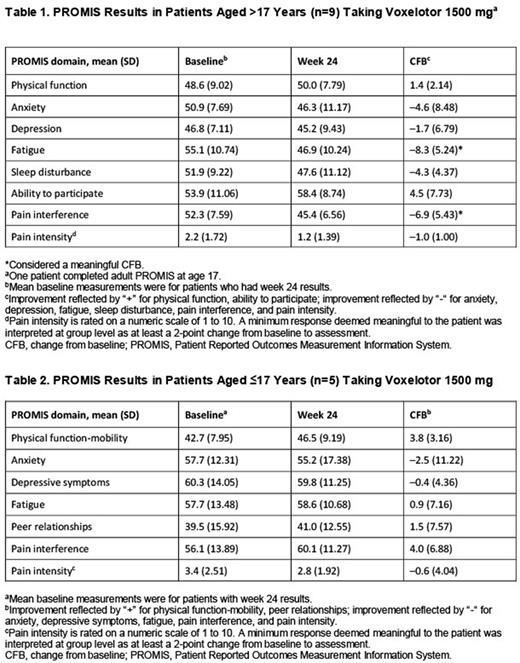
At week 24, 14 patients (9 adults, 5 adolescents) completed PROMIS measures; improvements in the mean CFB were reported across several PROMIS health domains. In adults, a meaningful CFB was found for both fatigue and pain interference. Some domains reached near-meaningful improvements, including anxiety, sleep disturbance, and ability to participate (in social roles/other activities), and others improved more modestly (Table 1). While some domains improved among adolescents, none reached a meaningful level (Table 2).
A total of 18 (72.0%) patients had ≥1 non-SCD-related TEAE, and 3 (12.0%) patients had ≥1 grade 3 non-SCD-related TEAE. No non-SCD TEAEs of grade 4 or higher were reported. The most common non-SCD-related TEAEs (>10%) were diarrhea (32.0%), headache (20.0%), nausea (20.0%), and vomiting (12.0%).
Conclusions: Over three-quarters of voxelotor-treated patients achieved an increase in Hb at week 24, and most patients and clinicians reported improvement in patients' overall health status. The PROMIS data in voxelotor-treated adults provided insights into improvements in general well-being, such as fatigue, pain interference, and ability to participate; most notable were fatigue and pain interference, both of which reached a CFB threshold considered meaningful. The safety profile reported in ActIVe is consistent with previous studies of voxelotor treatment. Overall, results suggest that voxelotor-treated patients may experience improvements in QOL, including in their physical and emotional well-being.
Funding: Global Blood Therapeutics.
Disclosures
Shah:Alexion: Speakers Bureau; CSL Behring: Consultancy; Novartis: Research Funding, Speakers Bureau; Bluebird Bio: Consultancy; Global Blood Therapeutics: Consultancy, Research Funding, Speakers Bureau. Brown:Novartis: Consultancy, Research Funding; Pfizer: Research Funding; Forma Therapeutics: Research Funding; Global Blood Therapeutics: Consultancy, Current Employment, Current equity holder in publicly-traded company, Research Funding; Imara: Consultancy, Research Funding; Novo Nordisk: Consultancy. Andemariam:Emmaus: Consultancy, Membership on an entity's Board of Directors or advisory committees; Aruvant: Consultancy, Membership on an entity's Board of Directors or advisory committees; Forma Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; CRISPR Therapeutics AG: Consultancy, Membership on an entity's Board of Directors or advisory committees; Global Blood Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Hemanext: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; Shenox: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi Genzyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; Terumo BCT: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novo Nordisk A/S: Consultancy, Membership on an entity's Board of Directors or advisory committees; Vertex: Consultancy, Membership on an entity's Board of Directors or advisory committees. Idowu:Novartis: Membership on an entity's Board of Directors or advisory committees; Pfizer: Research Funding; Forma: Research Funding; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Ironwood: Research Funding. Glaros:Global Blood Therapeutics: Other: Advisory Board; Bausch: Membership on an entity's Board of Directors or advisory committees. Glassberg:Synforma: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy; CSL Behring: Consultancy. Moehring:Global Blood Therapeutics: Current Employment, Current equity holder in publicly-traded company. Hoppe:Global Blood Therapeutics: Current Employment, Current equity holder in publicly-traded company. Dixon:Global Blood Therapeutics: Current Employment, Current equity holder in publicly-traded company. Manwani:Pfizer: Consultancy; Novartis: Consultancy; Global Blood Therapeutics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal